Smart CAPA management through RFID sample tracking for pharmaceutical quality assurance
When it comes to the safety, quality and traceability of production processes, the requirements in hardly any industry are as high as in the pharmaceutical industry.
Comprehensive documentation requirements and many other legal requirements must be complied with at all times. Accordingly, the highest quality standards are applied to data quality and sample tracking.
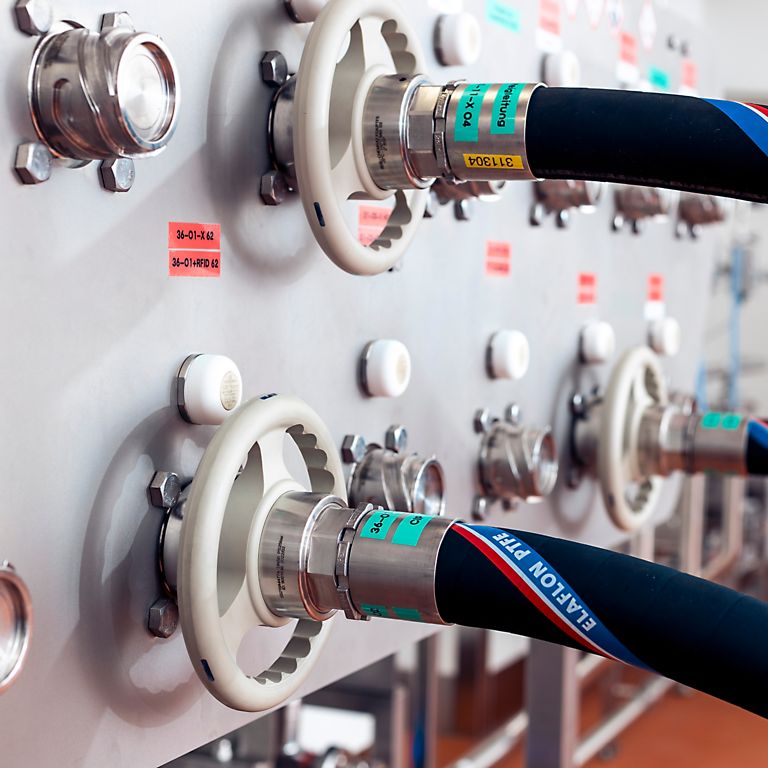
Regulatory authorities such as the FDA conduct unannounced inspections of pharmaceutical production. Their sanctions for regulatory violations range from the dispatch of the so-called "FDA Warning Letter" to the prohibition of a drug. With increasingly complex medical products, corrective and preventive action (CAPA) helps to ensure the highest quality standards in the pharmaceutical industry. Contactless identification solutions based on RFID enable high safety and quality standards to be maintained and seamless documentation to be implemented without the expense and error sources of manual systems – both in existing and new plants.
TURCK's RFID systems allow you to achieve consistent sample tracking and optimize your production processes. In addition, you ensure compliance with the ICH Q10 Guideline, which describes the requirements for a comprehensive pharmaceutical quality system and also includes the requirements of Good Manufacturing Practice (GMP). You get complete transparency through real-time data to make your production safe, efficient and compliant.
TURCK's digital automation technologies ensure maximum data integrity. Results outside the specification are thus ruled out: reproducible product quality, sustainable CAPA (corrective and preventive actions) and faultless GMP!